Abstracts from the 29^ National Conference of the Italian Society for the Study of Hemostasis and Thrombosis, Bergamo, Italy | 23-25 October 2025
Vol. 4 No. s1 (2025)
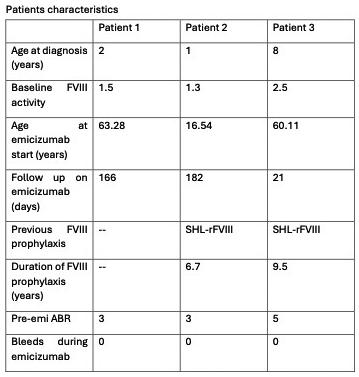
PO86 | Emicizumab in patients with moderate hemophilia A and severe bleeding phenotype: single-center real-world experience
A.L. Faccini, M. Biglietto, S. Sorella, E. Crisanti, M. Antonacci, R. Mormile, R. Ciciani, E. Baldacci, C. Santoro, S. Ligia | Hematology, Department of Translational and Precision Medicine Sapienza University of Roma, Italy
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Published: 22 October 2025
507
Views
0
Downloads
Similar Articles
- PO04 | Classification criteria for antiphospholipid syndrome: ACR/EULAR 2023 versus Sydney 2006 – A monocentric and retrospective study , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO38 | Rates of hemorrhagic events and hemodialysis circuit thrombosis in patients with end-stage renal disease on hemodialytic treatment receiving a factor-XI inhibitor: a systematic review and meta-analysis , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO66 | Viscoelastic tests in disseminated intravascular coagulation associated with aortic aneurysm , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO05 | Intensive FVIII replacement in haemophilia patients with hypertrophic synovium: a randomized study , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO39 | Bleeding events in Von Willebrand disease type 1 yearly treatable with desmopressin , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO67 | Pancreatic cancer in a 71-year-old severe hemophilia A patient with inhibitors and sustained zero bleeding during emicizumab prophylaxis , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO06 | Extracellular vesicles profiles in patients with porto-sinusoidal vascular disease , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO40 | Management of paediatric patients affected by mild factor VII deficiency undergoing surgery , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO68 | Whole blood hypercoagulable profiles in a patient with markedly elevated lipoprotein(A) plasma levels and thrombotic complications: a case report , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO07 | Liver transplant in a case of congenital afibrinogenemia , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
1-10 of 96
Next
You may also start an advanced similarity search for this article.