Abstracts from the 29^ National Conference of the Italian Society for the Study of Hemostasis and Thrombosis, Bergamo, Italy | 23-25 October 2025
Vol. 4 No. s1 (2025)
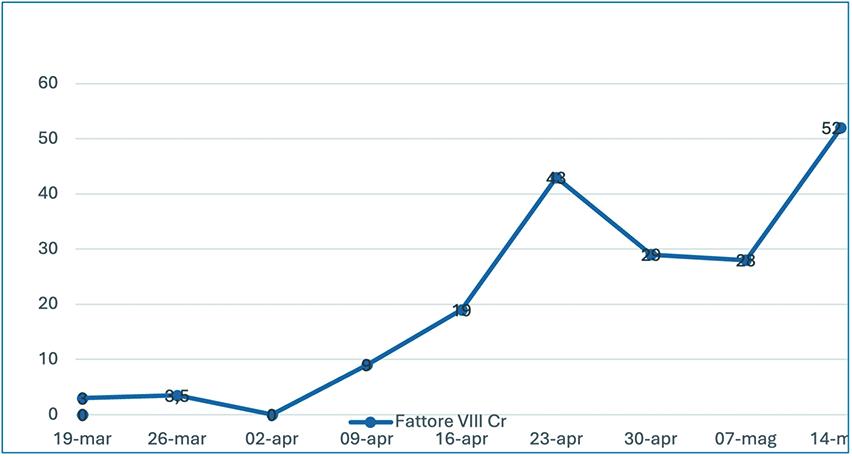
PO65 | Utilization of emicizumab in acquired hemophilia A: a case report
C. Caputo, P. Conca, I.L. Calcaterra, E. Cimino, M. Romeo, M. Aversano, E. Franco, C. De Luca, G. D’errico, L. Jr Valletta, M. Di Minno, A. Tufano | Department of Clinical Medicine and Surgery, Federico II University, Naples, Italy
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Published: 22 October 2025
381
Views
0
Downloads
Similar Articles
- PO48 | Thrombotic risk evaluation and thrombophilia testing in β-thalassemia patients: a retrospective cohort analysis from a reference regional center , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO81 | Direct oral anticoagulants in atypical site vein thrombosis: a single centre experience focused on cancer patients , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO20 | Impact of endovascular treatment on functional outcome in ischemic stroke: a real-world retrospective study , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO49 | Use of emicizumab in two patients with acquired hemophilia A: a new therapeutic approach , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO82 | Retrospective application of the TH2 score in a multicenter cohort of ITP patients: a reliable predictive tool or a score in need of refinement? , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO21 | Exploring endothelial damage: the interplay between coagulopathy, capillary leak and vasoplegia in sepsis , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO22 | Early detection and management of PICC-related thrombosis in oncohematologic patients through serial ultrasound monitoring: a prospective observational study , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO50 | Treatment with BTK inhibitors in patients affected by chronic lymphocytic leukemia: safety and hemostasis , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO83 | A case of deep vein thrombosis in a patient with systemic mastocytosis , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO23 | Plasmic score applicability for the diagnosis of thrombotic microangiopathy associated with acquired ADAMTS13 deficiency in emergency settings at the hub laboratory of AUSL Romagna: state of the art and future prospects , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
1-10 of 96
Next
You may also start an advanced similarity search for this article.