Abstracts from the 29^ National Conference of the Italian Society for the Study of Hemostasis and Thrombosis, Bergamo, Italy | 23-25 October 2025
Vol. 4 No. s1 (2025)
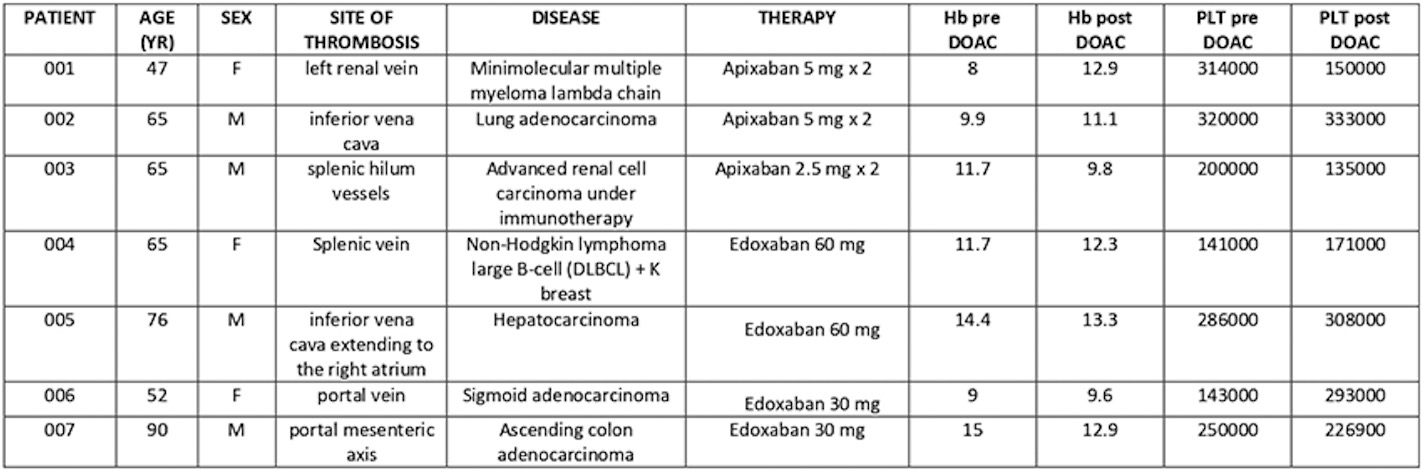
PO81 | Direct oral anticoagulants in atypical site vein thrombosis: a single centre experience focused on cancer patients
U. Mazzarelli1, A. Guida1, L. Agrelli1, M. Amato1, R. Guarrasi2, L. Santaniello2, C. Cardamone1, P. Vitale1, M. Triggiani1 | 1Centro per le Malattie Emorragiche Congenite e Malattie Trombotiche UOC Clinica Immunologica e Reumatologica AOU S. Giovanni di Dio e Ruggi d’Aragona, Salerno; 2UOC Clinica Oncologica AOU S. Giovanni di Dio e Ruggi d’Aragona, Salerno, Italy
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Published: 22 October 2025
448
Views
0
Downloads
Similar Articles
- PO28 | Long term effectiveness and safety of venous thromboembolism with fondaparinux: data from the start registry , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO56 | Safety of anticoagulant treatment in patients with splanchnic vein thrombosis and history of portal hypertension-related bleeding , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO89 | Apixaban for secondary prevention of venous thromboembolism in hemodialysis patients: pharmacokinetic profile and safety outcomes , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO29 | Quality of anticoagulation as a predictor of early left ventricular thrombosis resolution: a retrospective cohort study , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO57 | Direct oral anticoagulant determination in urine samples compared to plasma concentrations in outpatients with atrial fibrillation and obesity , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO90 | Acquired hemophilia A with high-titer inhibitor in a patient with cardiovascular and metabolic comorbidities , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO30 | Acquired angioedema associated with B-non Hodgkin lymphoma: a case report , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO58 | Gender differences in primary haemostasis , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO91 | Disseminated intravascular coagulation with deep vein thrombosis in a frail patient: an extreme manifestation triggered by sepsis during SGLT2i therapy , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO31 | Comparative analysis of anticoagulant therapy in patients over 90 years old: vitamin K antagonists versus direct oral anticoagulants , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
1-10 of 96
Next
You may also start an advanced similarity search for this article.