Abstracts from the 29^ National Conference of the Italian Society for the Study of Hemostasis and Thrombosis, Bergamo, Italy | 23-25 October 2025
Vol. 4 No. s1 (2025)
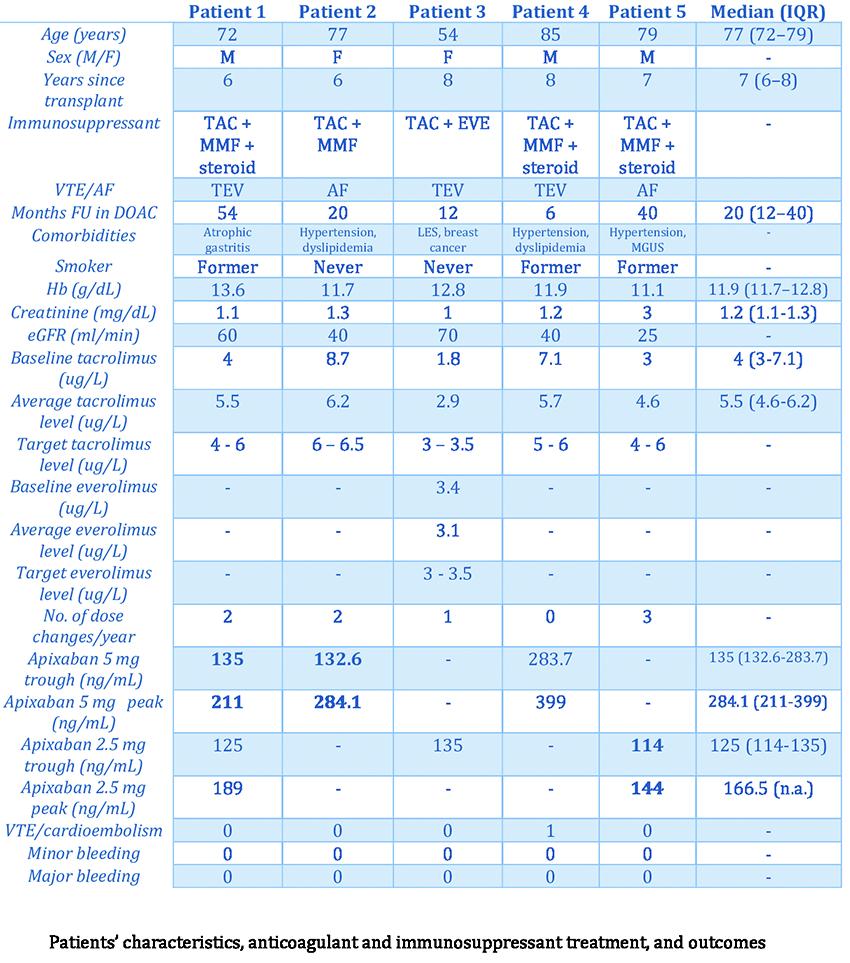
PO70 | Clinical safety and monitoring of apixaban combined with tacrolimus and everolimus in kidney transplant patients: a prospective study
S. Guglielmo1, E. De Bon2, L. Scarano1, R. Fabris1, E. Pierobon3, S. Barbar2 | 1Internal Medicine, Department of Medicine, Azienda ULSS 6 Euganea, Cittadella; 2Thrombotic and Hemorrhagic Disorders Unit, Department of Medicine, Azienda ULSS 6 Euganea, Cittadella; 3Nephrology and Dialysis Unit, Azienda ULSS 6 Euganea, Cittadella and Camposampiero, Padova, Italy
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Published: 22 October 2025
400
Views
0
Downloads
Similar Articles
- PO30 | Acquired angioedema associated with B-non Hodgkin lymphoma: a case report , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO58 | Gender differences in primary haemostasis , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO91 | Disseminated intravascular coagulation with deep vein thrombosis in a frail patient: an extreme manifestation triggered by sepsis during SGLT2i therapy , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO31 | Comparative analysis of anticoagulant therapy in patients over 90 years old: vitamin K antagonists versus direct oral anticoagulants , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO59 | Efficacy and safety of different anticoagulant treatment regimens for isolated internal jugular vein thrombosis: a case series , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO92 | Challenging management of portal vein thrombosis in a cirrhotic patient on hormone therapy after breast cancer , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO32 | Effect of the sodium-glucose co-transporter-2 (SGLT2) inhibitors on platelet activation and lipid peroxidation: results of a pilot study , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO60 | Asymptomatic PNH clone in acquired aplasia (AA) , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO93 | Use of DOACS in heart transplantation recipients , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO33 | Whole blood hypercoagulable profiles in patients with rheumatoid arthritis , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
1-10 of 96
Next
You may also start an advanced similarity search for this article.