Abstracts from the 29^ National Conference of the Italian Society for the Study of Hemostasis and Thrombosis, Bergamo, Italy | 23-25 October 2025
Vol. 4 No. s1 (2025)
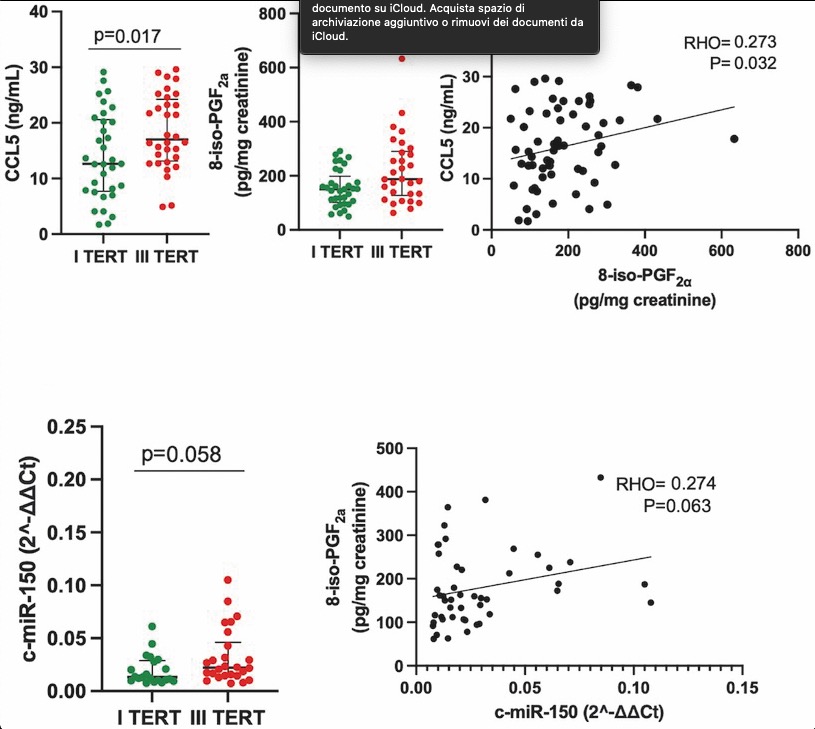
CO42 | Oxidative stress may contribute to enhanced thrombopoiesis through miR-150: implications for aspirin response
R. Liani1, P.G.M. Simeone1, M. Alfonsetti1, M. Frezza1, R. Tripaldi1, S. Ciotti1, M. Luongo2, F. Cipollone1, L. Vanella3, F. Santilli1 | 1Department of Medicine and Aging, and Center for Advanced Studies and Technology “CAST”, G. d’Annunzio University of Chieti-Pescara, Chieti; 2Immunotransfusion Service, Clinical Haematology of Chieti University Hospital, Chieti; 3Department of Drug Sciences, University of Catania, Italy
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Published: 22 October 2025
365
Views
0
Downloads
Similar Articles
- CO43 | Early platelet dysfunction in sepsis: an ICU pilot study , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- Silvia Linari, Renato Marino, Marzia Leotta, Antonio Coppola, Patrizia Di Gregorio, Augusto Bramante Federici, Flora Peyvandi, Cristina Santoro, Ezio Zanon, Rita Carlotta Santoro, The landscape of rare coagulation factor deficiency management in Italy: a national hemophilia center survey , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. 3 (2025)
- CO40 | The co-inheritance of two ITGB3 variants exerting additive detrimental effects on platelets leads to variant Glanzmann thrombasthenia , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- Alice Lipari, Esmeralda Capristo, Antonietta Ferretti, Erica De Candia, Anticoagulation in obese patients: challenges and strategies , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. 3 (2025)
- CO45 | Role of urinary 11-dehydro-thromboxane B2 as a predictor of preeclampsia in pregnant high-risk women on low-dose aspirin treatment , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- CO46 | Comparison of reticulated platelet procoagulant role in liver cirrhosis vs. fibrosis , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- CO44 | First interlaboratory validation workshop of immunofluorescence microscopy on the peripheral blood smear for recognizing patients with inherited platelet disorders , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- CO47 | Genetic characterization of the KIV2 LPA polymorphism in subjects with bicuspid aortic valve with different clinical complications , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- CO50 | Diagnostic accuracy of the heparin-induced multiple electrode aggregometry (HIMEA) functional test for heparin-induced thrombocytopenia , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- CO51 | Novel biomarkers of neurological damage in acute immune-mediated thrombotic thrombocytopenic purpura , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
1-10 of 127
Next
You may also start an advanced similarity search for this article.