Abstracts from the 29^ National Conference of the Italian Society for the Study of Hemostasis and Thrombosis, Bergamo, Italy | 23-25 October 2025
Vol. 4 No. s1 (2025)
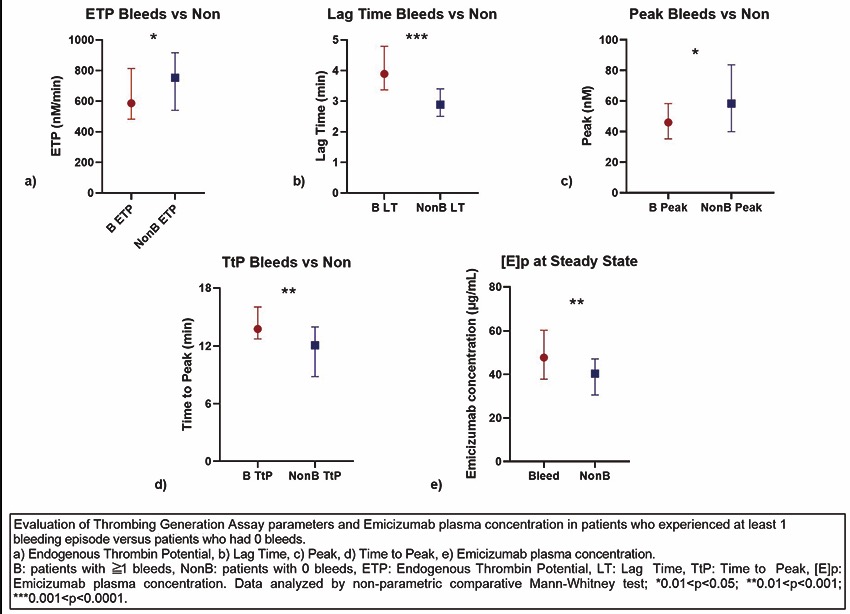
CO34 | Evaluation of emicizumab concentration and thrombin generation in a cohort of severe hemophilia A patients with and without inhibitor
A. Taglietti1, S. Ligia1, F. Paoletti1, R. Mormile1, E. Baldacci2, A. Chistolini1, C. Santoro1,2 | 1Hematology, Department of Translational and Precision Medicine, Sapienza University of Roma; 2Hematology, Azienda Ospedaliera Universitaria Policlinico Umberto I, Roma, Italy
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Published: 22 October 2025
398
Views
0
Downloads