Abstracts from the 29^ National Conference of the Italian Society for the Study of Hemostasis and Thrombosis, Bergamo, Italy | 23-25 October 2025
Vol. 4 No. s1 (2025)
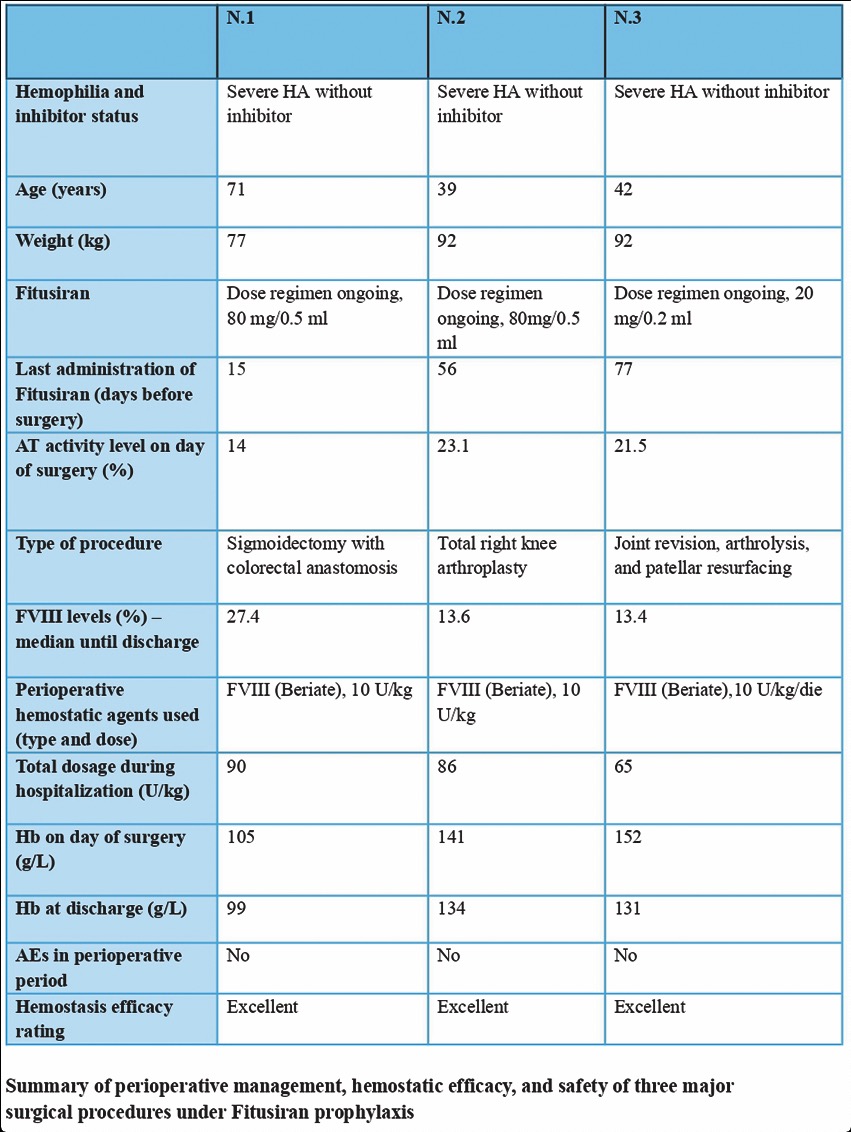
CO32 | Major surgery in patients with severe hemophilia A without inhibitors receiving prophylaxis with fitusiran
E. Zanon, C. Simion, A. Napolitano, L. Spiezia, P. Simioni | Haemophilia Center, First Chair of Internal Medicine, Department of Medicine, University-Hospital of Padua Medical School, Padua, Italy
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Published: 22 October 2025
358
Views
0
Downloads
Similar Articles
- Silvia Linari, Renato Marino, Marzia Leotta, Antonio Coppola, Patrizia Di Gregorio, Augusto Bramante Federici, Flora Peyvandi, Cristina Santoro, Ezio Zanon, Rita Carlotta Santoro, The landscape of rare coagulation factor deficiency management in Italy: a national hemophilia center survey , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. 3 (2025)
- CO35 | Bleeding phenotype characterization in patients with congenital bleeding disorders: a multigenic approach using next generation sequencing to identify coagulation-modulating variants , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- CO33 | Acquired hemophilia A in Liguria, Italy: what six years of data reveal , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- CO38 | Management of pregnancy, delivery, and postpartum in Italian carriers and women with hemophilia A and B , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- CO36 | Inhibitor titer at diagnosis and clinical course in acquired hemophilia A: a single-center experience , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- CO27 | Intracerebral hemorrhage in hemophilia A and B: a 10-year prospective multicenter study in Italy (2012–2022) , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- CO37 | Assessment of clotting factor activity and sterility in thawed fresh frozen plasma supplied to helicopter emergency medical services for prehospital transfusion , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- CO34 | Evaluation of emicizumab concentration and thrombin generation in a cohort of severe hemophilia A patients with and without inhibitor , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- CO29 | Emergency room physicians and oral anticoagulants related bleeding , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- CO30 | Joint prosthetic surgery in patients with hemophilia A: clinical-functional outcomes and quality of life , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
1-10 of 133
Next
You may also start an advanced similarity search for this article.